
Angelica Root
Angelica root, known as Norwegian angelica or garden angelica, comes from the family of Apiaceae, which also includes carrots and celery. The scientific name of this plant is Angelica archangelica and it grows natively in Syria; however, it has spread in many parts of Europe and western Asia. As the plant became more commonly used, it began to spread southward into Europe, where eventually, it picked up the name - the “angel” plant.
Introduction
The dried roots are frequently used for flavoring cakes, candy, and beverages, such as gin, and are used in perfumery for its musky scent [1]. It has had a strong impact on Nordic culture, as it is believed to possess antimicrobial and antibacterial properties. As such, it has been incorporated in the diets of the Sami population, the Indigenous population of the respective area [2].
Through extraction of the plant’s roots, an essential oil can be obtained. The chemical composition of the essential oil contains several terpenes, which are a class of simple hydrocarbons that are often considered to be biologically active and contribute to a plant’s aroma. The most abundant terpenes found in the essential oil of angelica root include, α-pinene at 29.7%, β-phellandrene + limonene at 13.2%, and δ-3-carene at 14.2% [3]. The presence of these compounds is indeed confirmed by several publications which focus heavily on the extraction of the fresh roots using a variety of techniques. This research, however, was more so concerned about the commercial product of angelica root, which comes to you dried and ready to use to make, for example, a tea. The fresh roots, on the other hand, are not sold commercially.
[1] Charles, D. J. (2012). Angelica. Antioxidant Properties of Spices, Herbs and Other Sources, 151–157. https://doi.org/10.1007/978-1-4614-4310-0_7
[2] Toneu, T., Kjesrud, I., Karoline, Kool, & Anneleen. (2020). Sweetness Beyond Desserts: The Cultural, Symbolic, and Botanical History of Angelica (Angelica archangelica) in the Nordic Region. Source: Journal of Ethnobiology, 40(3), 289–304. https://doi.org/10.2993/0278-0771-40.3.289
[3] Aćimović, M. G., Pavlović, S. D., Varga, A. O., Filipović, V. M., Cvetković, M. T., Stanković, J. M., & Čabarkapa, I. S. (2017). Chemical composition and antibacterial activity of Angelica archangelica root essential oil. Natural Product Communications, 12(2), 205–206. https://doi.org/10.1177/1934578X1701200216

Purpose
Some considerations and questions that this project sought to explore were, firstly, the differences between the extraction of the fresh roots versus the extraction of the dried roots. In other words, is the chemical composition consistent with the consumer product (the dried roots) compared to the fresh roots? Secondly, which extraction technique allows us to see the most biologically active compounds, and which is most effective in performing this task based on efficiency and yield? Finally, do the dried roots have any solid biological basis to claim, as per the manufacturer, the proposed medicinal properties associated with the fresh roots, including “greatly healing, especially for women” and “a healing herbal tea”?

Extraction Methods (Solvent Extraction)
The first extraction technique used was solvent extraction, where the dried angelica root was first obtained through Amazon via a company called Valley of Tea. The three solvents that was used to perform the extraction with was then collected, which were ethyl acetate, hexanes, and chloroform. 15 mL of each solvent was placed into separate vials with approximately 5.0 grams of plant material added to each one, which had first been ground into a fine powder. The vials were then thoroughly shaken with a mechanical shaker for about 15 minutes, filtered, and underwent GC-MS analysis.

Extraction Methods (Supercritical CO₂ Extraction)
The second extraction technique used was supercritical CO2 extraction, which refers to CO2 having no definite liquid or gas phase. In essence, supercritical CO2 has a gas-like diffusion rate with a liquid-like solvent density. The extraction involves a specialized machine that works in the following way;
1) First, finely ground plant material – in this case, angelica root – is placed into an extractor chamber.
2) Then, there is a pump that forces pressurized CO2 into the extractor chamber at a desired temperature and in the supercritical state.
3) Once in the chamber, the supercritical CO2 acts as a solvent with the angelica root, dissolving the chemical compounds that it contains.
4)It then carries the extract past a pressure release valve and into a separator.
5/6) Once in the separator, the pressure lowers and the CO2 reverts into a gas, separating itself from the extract.
7) The extract can then be collected in a collection vessel, such as a vial, to be used for further analysis.
8) The CO2, in the meantime, gets routed into an exhaust to complete the process.
Approximately 50 grams of plant material was added to the machine and the extract obtained was slightly less than 1 gram of angelica root.

Data (Essential Oil Composition)
To address the first research question, the chemical composition of the essential oil (EO), which is what you will receive when you perform an extraction of the fresh roots through distillation. The chromatogram of a sample of the EO of angelica root obtained via Aromatics is shown below. The temperature gradient used was from 40 degrees - 250 degrees. The most abundant molecules here are α-pinene at approximately 27%, δ-3-carene at approximately 17%, and β-phellandrene at approximately 22%, which matches with other research publications.

The other peaks can be identified and are described in Table 1.

Data (Solvent Extraction - Ethyl Acetate)
As previously discussed, solvent extraction was performed with 3 different solvents. As an example, the extraction with ethyl acetate as the solvent is presented below. The temperature gradient was from 150 degrees to 310 degrees as performing a run from 40 degrees to 250 degrees did not allow for the identification of molecules in the lower temperature area, but there were some peaks in the higher temperature area. A higher temperature gradient produced the appearance of molecules such as osthole, the most abundant, at approximately 24%, methoxsalen at approximately 2%, and β-bisabolene at approximately 1%. The other molecules with significantly larger peaks could not be identified regardless of method used, which contrasts significantly with the essential oil sample.
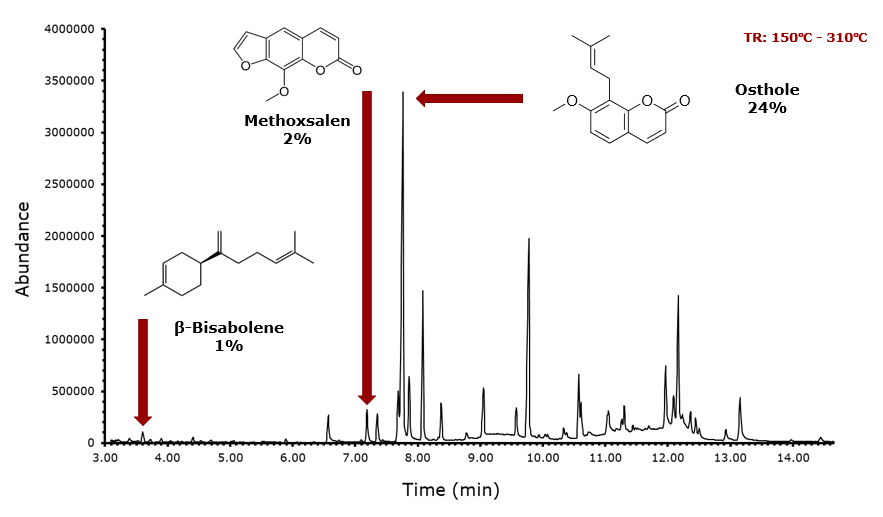
Data (Supercritical CO2 Extraction)
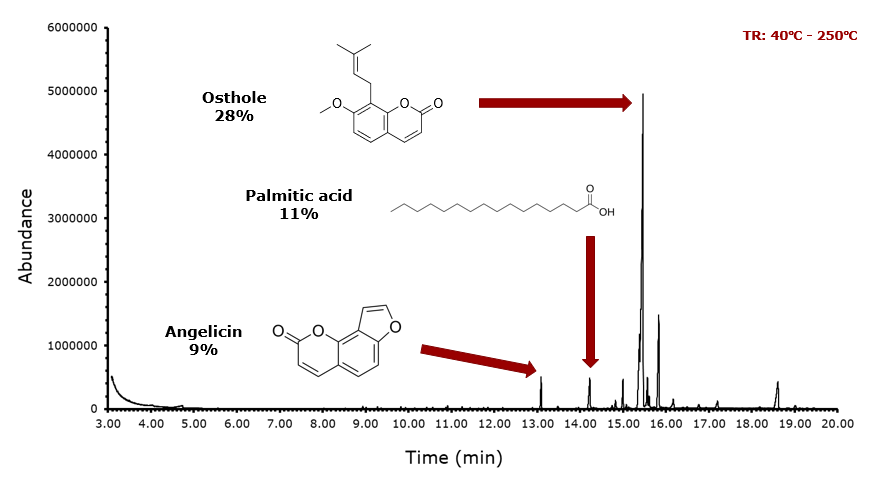
With respect to the supercritical CO2 extract, it was first dissolved in 1 mL of ethyl acetate as it could not have been run directly due to its paste-like state. The temperature ramp for this trial was from 40 degrees to 250 degrees. As with the ethyl acetate extract, osthole was the most abundant molecule at approximately 28%, but we see a jump in abundance with palmitic acid with approximately 11% and the appearance of angelicin at approximately 9%. Though angelicin is a molecule almost exclusive to this species, it has only been found in this extract and not in any other extract. Interestingly, there appeared to be some minor peaks within the 9-minute and the 12-minute range, so to isolate this area, another trial was run with a shorter temperature range.
The sample itself was unchanged – it was still dissolved in 1 mL of ethyl acetate, but this time, the temperature range was between 80 degrees to 150 degrees. Though the chromatogram looks busy, there is some useful information to be taken from this. Carvacrol, a terpenoid, was identified at 5%, thymoquinone, a phytochemical considered to be an aspiring natural drug, was found with an abundance of 2%, and finally, α-copaene, a sesquiterpene, was identified at 7%. These molecules have not been found in any previous extracts and carvacrol and thymoquinone have not been reported to be found in the fresh roots of angelica root whatsoever.

Analysis (Fresh Roots vs. Dried Roots)
The initial hypothesis was that the dried roots would contain terpenes as identified in the fresh roots. Instead, what we saw were molecules that all looked similar to each other, including methoxsalen, isopimpinellin, and psoralene, where the latter two were present in minute abundance in certain extracts. All the molecules are derived from coumarin, an aromatic compound with a sweet odor found in high concentrations in cinnamon. The most abundant of these compounds was osthole, forming a considerable part of the composition of the dried roots.

The commonality with the compounds in the dried roots is that they all have relatively high boiling points compared to the fresh roots. If we compare the boiling points of these compounds with the molecules found in the essential oil (shown in red), you can certainly see that they are quite drastically far apart. Considering how essential oils are extracted, this remark makes sense as the volatile compounds would have had to come up with water during the distillation process, so it would be unlikely that the essential oil would contain compounds with high boiling points.

Analysis (Extraction Methods)
With respect to the extraction methods, the first point to make is that despite the use of different solvents, similar results were obtained. Figure 10 shows the chromatograms of the trials with all the solvents overlayed. The positioning of each of these peaks is identical and consistent with each solvent with only minor differences in the relative heights of peaks.
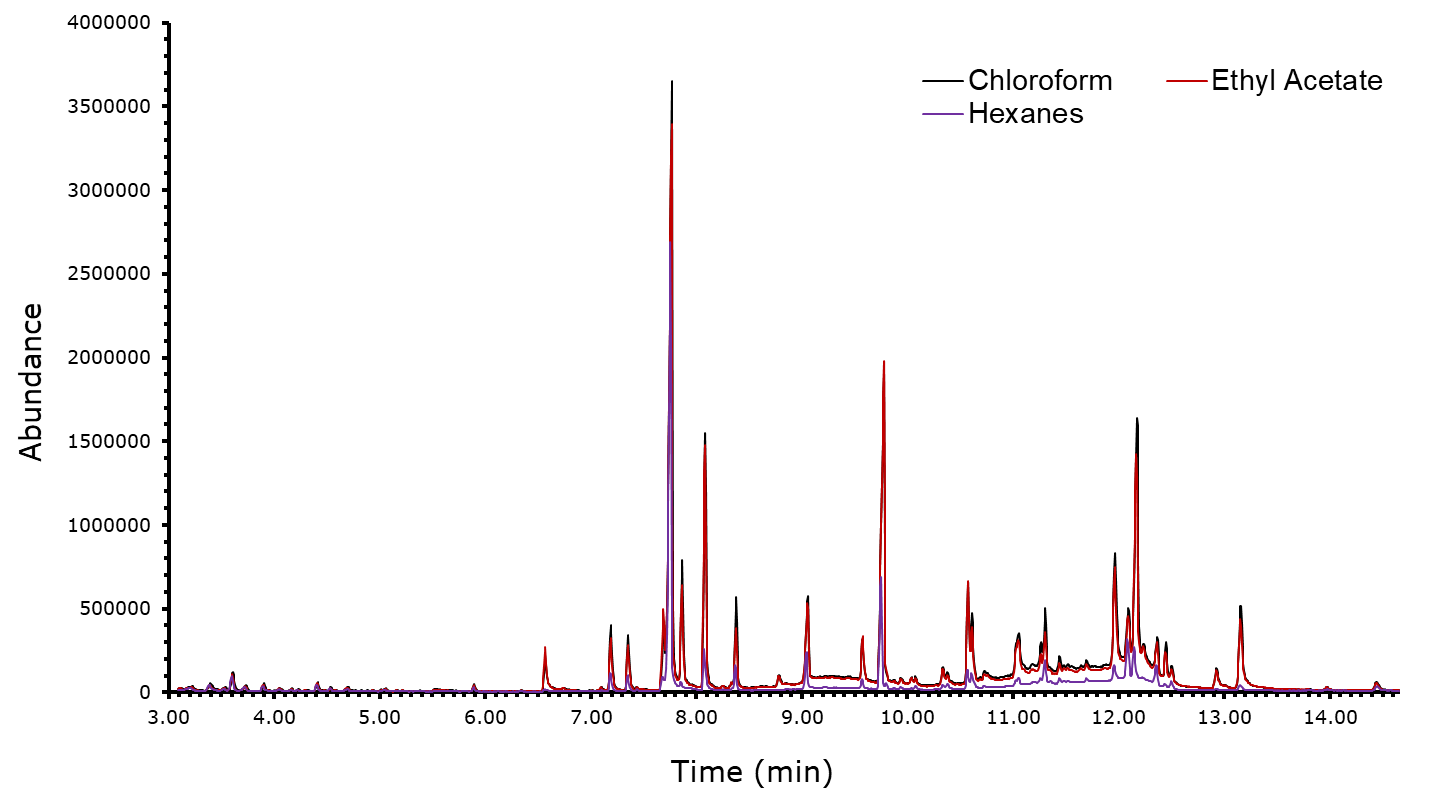
On the other hand, the supercritical CO2 extraction method allowed us to see molecules that were not previously identified, either in former publications or in other extracts. These molecules include, angelicin, carvacrol, and a-copaene. Not performing this extraction would have resulted in these compounds getting overlooked. Though it seems as though supercritical CO2 extraction is the most feasible technique, the yield of this process must be considered. A considerable amount of plant material was added to the machine for an extract that was not great in quantity. If obtaining enough material is not a problem, then this would not be a concern, but if it is, this is something to keep in mind.

Conclusion
To summarize, the primary objective of this research was to analyze the dried roots of Angelica archangelica as the consumer product as it has been marketed based on several research publications about the chemical composition of the fresh roots and its proposed antimicrobial and antibacterial properties. It became clear that the dried roots did not contain the same compounds as you would see with an extraction of the fresh roots, notably the lack of the biologically active terpenes believed to be responsible for the plant’s medicinal qualities. Instead, what was observed was a considerable abundance of coumarin derivatives with the most abundant of which being osthole. The question surrounding the most effective extraction technique was also addressed with supercritical CO2 extraction identifying compounds that would have otherwise been overlooked with solvent extraction. One does have to take into consideration the yield with this method, but it certainly doesn’t detract from this advantage. Finally, does the manufacturer’s claims of the dried roots having great healing properties hold true? Doubtful, as the terpenes seemingly responsible for these properties of the plant have likely been lost during the industrial heating process.

About Me
My name is Monica, and I'm volunteering at the John Holmes Mass Spectrometry Laboratory for the summer of 2024. I’m heading into my second year at the University of Ottawa, where I’m doing an undergraduate degree in biomedical science. The environment in the lab has afforded me the ability to continue my academic career in the sciences, and the experience has opened my eyes to the world of analytical chemistry!
