It can therefore be concluded that the microwave distillation method makes it possible to have hydrosols containing a higher concentration of carvone. In addition, thanks to this second method we can say that peppermint has a larger portion of carvone. To conclude, we also note that the external calibration method allowed to have a very good 𝑅2 unlike the internal calibration method.

Menthe poivrée et menthe verte
Plants play a crucial role as primary producers in ecosystems, converting solar light into food through photosynthesis, essential for meeting fundamental needs such as food, clothing, shelter, and medicine. With the increasing global population, incomes, and urbanization, the demand for these resources continues to grow.
Presentation
Hi, my name is Amina, and I am 21 years old. I'm in my second year of pursuing a Bachelor's degree in Chemical Engineering with a specialization in Analytical Chemistry at the University of Montpellier in France.
Currently, I am undertaking my second-year internship at the University of Ottawa. This choice aligns with my career aspirations in the cosmetics and perfumery industry. The focus of my internship revolves around hydrosols, which complements my academic journey perfectly.
During this internship, I will be conducting GC-MS analyses on these hydrosols, allowing me to deepen my understanding of analytical techniques in this field.

The mint family
The Lamiaceae family, primarily found in temperate regions, provides a fertile ground for studying plant adaptation to specific environments. Among them, the genus Mentha stands out with emblematic species such as Mentha spicata (spearmint) and Mentha x piperita (peppermint).
Spearmint is valued for its culinary use and digestive and soothing properties. In contrast, peppermint, rich in menthol, is renowned for its stimulating, antispasmodic, and analgesic effects, widely used in aromatherapy and pharmaceutical products. Despite their close botanical relationship, they offer diverse applications due to their unique compositions and effects.
My ultimate goal for this project is to analyze the hydrosols of spearmint and peppermint to determine their constituent compounds. This analysis will enable me to assess the effects of these hydrosols and identify their potential applications in cosmetics, food, and pharmaceuticals.

GC/MS Analysis
Analyzing method for essential oils
Injector :
- Volume : 1 μL
µ µ
Inlet :
- Heater : 200°C
Oven
- Initial temperature : 40°C
- Final temperature : 300°C
- Ramp : 15°C/min
Analyzing method for fragrance oils
Injector :
- Volume : 1 μL
Inlet :
- Heater : 250°C
Oven :
- Initial temperature : 40°C
- Final temperature : 300°C
- Ramp : 15°C/min

Analyzing method for hydrosols
Injector :
- Volume : 1 μL
Inlet :
- Heater : 200°C
Oven :
- Initial temperature : 40°C
- Final temperature : 250°C
- Ramp : 15°C/min
Analyzing method for the headspace
Injector :
- Volume : 1 μL
Inlet :
- Heater : 200°C
Oven :
- Initial temperature : 40°C
- Final temperature : 300°C
- Ramp : 15°C/min

Peppermint
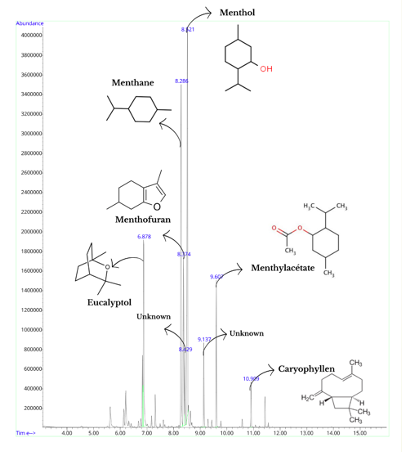
Analysis : Peppermint essential oil
Before producing hydrosols from both plants, I analyzed their essential oils to gain insight into the chemical compounds that might be present in the hydrosols.
Upon comparing the chromatograms of the essential oils extracted from both plants, I observed several common chemical compounds.
These include limonene, a terpene with a citrus scent known for its antimicrobial and anti-inflammatory properties, widely used in cosmetics and food flavoring.
Eucalyptol, another shared compound, is a cyclic ether and monoterpene known for its mint-like odor and antimicrobial effects, commonly used in cough suppressants, mouthwashes, and insect repellents.

Analysis : Peppermint essential oil
Menthane, a saturated cyclic hydrocarbon with a mild minty odor, was also present, utilized in the synthesis of menthol and perfumes.
Menthol itself, a monoterpenoid alcohol, stands out with its strong minty odor and cooling sensation, widely used in analgesics, oral hygiene products, and as a flavoring agent.
Menthyl acetate, an ester derived from menthol and acetic acid, was detected with its fruity and minty aroma, commonly found in fragrances, flavorings, and cosmetics.
Lastly, caryophyllene, a bicyclic sesquiterpene, contributes a spicy and woody aroma with anti-inflammatory and analgesic properties used in medicinal applications, flavorings, and perfumes.

The SPME of peppermint essential oil
The SPME revolutionizes the analysis of volatile and semi-volatile compounds in analytical chemistry. It employs a fiber or a solid coating at the tip of a probe. To perform SPME, I heat the sample I want to analyze, then I introduce the fiber into the headspace to absorb the volatile compounds present. These compounds are retained on the surface of the fiber through various physical and chemical interactions. Subsequently, the loaded fiber is introduced into the GC to analyze the retained chemical species. The essential oil was heated to 50°C. I left the SPME in the headspace for 5, 7, 10, and 14 minutes. I decided to keep the results obtained with a 14-minute sampling time because that's when I obtained the best results.

Analysis : The SPME of peppermint essential oil
After analysis, I identified the six major chemical species found in the essential oil. I obtain a chromatogram that remains similar to what I had for the essential oil. This means that these chemical species are highly volatile. We can therefore remember that exposure to this essential oil allows you to have all the benefits of the majority products that make it up. This is very intriguing because it suggests that simply inhaling this essential oil can offer significant health benefits.
Peppermint hydrosol
I produced two hydrosols for each plant using two different methods: microwave distillation and hydrodistillation.
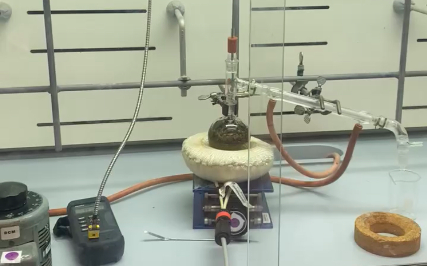
Microwave distillation
During my internship, I learned an innovative method for producing hydrosols developed by our lab. It's efficient and doesn't need specialized equipment. Here’s how it works: Plant material goes into a jar with a beaker and perforated funnel inside. An ice cube on the lid, microwaved in 7-minute sessions, helps distill hydrosol. Sessions continue until the odor diminishes, avoiding overheating. Water can be added for dry plants, aiding vapor-liquid exchanges to yield the hydrosol.

Hydrodistillation
Hydrodistillation is a widely used method to extract volatile compounds from plants. It involves placing plant materials in a specialized distillation vessel, typically a flask filled with water, and applying heat to vaporize aromatic compounds. Temperature control is crucial to prevent degradation of sensitive compounds while promoting efficient evaporation. The resulting steam, enriched with the plant's aromatic essence, is cooled to condense into a liquid state, forming a hydrosol or essential oil depending on the concentration of extracted compounds. This method is favored in industries for its ability to capture natural fragrances and therapeutic properties from botanical sources.
Analysis : Peppermint hydrosol
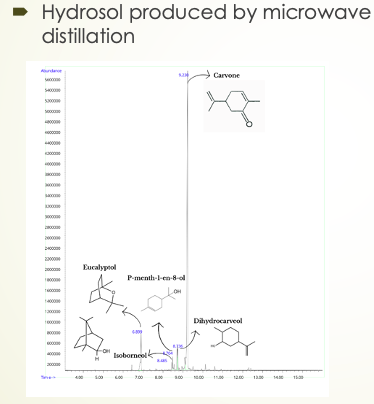
The chromatograms obtained with peppermint hydrosol are unexpected.
First of all, there is no signal of the presence of menthol, which is the main chemical species in essential oil. But what makes it even more amazing is that the plant that is peppermint is known for its menthol. It is the plant of the Mentha family that constitutes the largest contribution of menthol. So it is not only an inconsistency with the essential oil but an inconsistency with the composition of the plant itself.
I decided to add a new method to produce the hydrosol, which is hydrodistillation in order to check the reliability of my results.
Analysis : Peppermint hydrosol
Even with the hydrodistillation, I still cannot find menthol, and I observe that carvone is indeed the predominant product in the peppermint hydrosol.
Both methods yield new products. One notable compound is p-menth-1-en-8-ol, a monoterpene alcohol renowned for its potent antimicrobial properties. It features herbaceous and lemony notes that impart freshness and vitality, making it valuable in cosmetics, cleaners, and disinfectants. Its sensory appeal enhances the user experience while ensuring cleanliness and safety.
Another compound observed is dihydrocarveol, a terpene alcohol and hydrogenated derivative of carvone. It offers aromatic and antimicrobial properties with woody, herbaceous, and slightly spicy notes, adding olfactory complexity and health benefits to various products.
Carvone emerges as the primary product in both hydrosols, presenting in its R-carvone form in spearmint, known for its distinctive spearmint or caraway scent. Beyond its aromatic charm, carvone contributes antimicrobial properties and digestive benefits, making it sought after in food flavors, fragrances, and digestive health products. Its multifaceted qualities enrich products with freshness, depth, and functional benefits.

Spearmint
Analysis : Spearmint essential oil
I analyzed the spearmint essential oil. However, for this plant, I only had one brand. I found a total of 6 products ranging from 5.693 minutes to 10.922 minutes:
It can be seen, the presence of some products previously found in peppermint oil such as limonene, eucalyptol, carvone and caryophyllen. We expect to find their benefits in spearmint essential oil.
Then there is dihydrocarvone, a terpenoid ketone that gives off a minty smell. As an intermediate in the production of other flavors and fragrances, it is widely used in the flavor, fragrance, and pharmaceutical industry. Its presence is common in food flavors, fragrance compositions, and medicines, providing a touch of minty freshness and thus contributing to a variety of applications.

Analysis : The SPME of spearmint essential oil
I then analyzed the headspace of the essential oil, as for peppermint. I used the same settings by heating the oil to 50 °C and leaving the fiber in the headspace for 14 minutes.
I notice that in the headspace, there is a greater proportion of limonene than carvone. This can mean that if you heat the essential oil and smell it, you will have a smell that is more orange than minty. In addition, the benefits of carvone are greater than those of limonene so the heated and smelled essential oil will have a worse effectiveness than the essential oil itself, contrary to what we have seen before with peppermint.

Analysis : Spearmint hydrosol
Concerning spearmint hydrosols, the results are more consistent.
The results obtained with the two methods are identical. I think that the jasmone is indeed the unknown peak at 10.598 only that it was badly separated and that the ms could not identify it as for the second method. First of all, the majority product is carvone, something I was able to predict with the analysis of the essential oil.
Secondly, unlike peppermint, menthol is found in spearmint hydrosol. It is a chemical species that is present in small proportions in spearmint even if I did not find any in spearmint essential oil.
Finally, I discovered a new chemical species which is 1-terpinen-4-ol [21]. This compound, classified as a monoterpene alcohol, demonstrates a variety of promising pharmaceutical properties. Its antimicrobial and antifungal capability makes it an attractive candidate in the field of pathogen control. In addition, its anti-inflammatory effects are worthy of attention, especially in the context of research on skin treatments. The olfactory notes at the heart of the aromatic profile, characterized by a herbaceous and slightly spicy combination, add a special sensory dimension to any product in which it is incorporated.

Calibration method
Internal standard with 4-ethylguaiacol
For this calibration, I chose a linearity range between 0 and 1 μL/mL. I make 6 samples by introducing the appropriate volume of 4-ethylguaiacol into 1 mL of ethyl acetate. Then when I start the analysis, I program a repetition of 7 in order to have the most values.
Finally, using the curve equations, I calculate the concentration of each of the chemical species present in the hydrosols.
Peppermint
- Microwave distillation
Area (Carvone) = 150452039
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 1.2 𝑚𝐿/𝐿
- Hydrodistillation
Area (Carvone) = 95700611
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 0.66 𝑚𝐿/𝐿
Speamint
- Microwave distillation
Area (Carvone) = 122700595
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 0.93 𝑚𝐿/𝐿
- Hydrodistillation
Area (Carvone) = 53084042
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 0.23 𝑚𝐿/𝐿

Internal standard with 4-ethylguaiacol
I performed the external calibration with carvone since it is the chemical species present in both hydrosols and in very large proportions.J’ai realise un etalonnage allant de 0,025 a 1 μL/mL.
Finally, using the curve equations, I calculate the concentration of each of the chemical species present in the hydrosols.
Peppermint
- Microwave distillation
Area (Carvone) = 150452039
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 1.64 𝑚𝐿/𝐿
- Hydrodistillation
Area (Carvone) = 95700611
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 1.03 𝑚𝐿/𝐿
Speamint
- Microwave distillation
Area (Carvone) = 122700595
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 1.33 𝑚𝐿/𝐿
- Hydrodistillation
Area (Carvone) = 53084042
𝐶 (𝐶𝑎𝑟𝑣𝑜𝑛𝑒) = 0.56 𝑚𝐿/𝐿

Conclusion
In conclusion, peppermint and spearmint hydrosols offer many valuable applications in various fields. In the food industry, peppermint brings a refreshing flavor with menthol and herbaceous notes, ideal for drinks and desserts, while spearmint adds unique herbaceous and minty notes to varied products. In cosmetics, both hydrosols provide a feeling of freshness and have antimicrobial properties, helping to revitalize the skin and fight bacteria and impurities. Finally, in the pharmaceutical field, peppermint is useful for clearing the respiratory tract and preventing skin infections, while spearmint relieves muscle aches and headaches, while offering an antifungal action. Thus, these hydrosols demonstrate their versatility and value in the food, cosmetics and pharmaceutical industries

Since I didn't find menthol in peppermint hydrosol, it might be worth trying with fresh leaves and analyzing different parts of the plant. This approach could help determine whether menthol is concentrated in a specific part of the plant, such as the stems, which would explain its absence in my results.