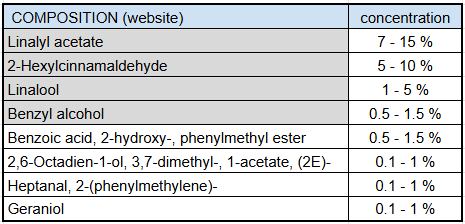
To conclude, we can see that the perfect balance of each chemical component allows to recreate the smell of jasmine flowers. The mix of natural and synthetic molecules brings back memories of hot summer days when the scent of flowers wafts across the Mediterranean coast.
Natural compounds are predominant because they made up the fragrance thanks to their smell properties. Each one is not necessarily added individually: we can suppose that linalool and linalyl acetate provide from the lavender essential oil. Also D-limonene, benzyl acetate, 2-hexyl cinnamaldehyde and methyl dihydrojasmonate are naturally present in the jasmine absolute.
Moreover, synthetic molecules allow to intensify the smell and stabilize the chemical composition over time to create a product with multiple uses.
According to our research, fragrance is a safe product. However, care must be taken when using this chemical product as it can cause irritation.