
Helichrysum hydrosol
Helichrysum italicum essential oil, part of the Asteraceae family, is known for its therapeutic properties. Extracted from the flowers through steam distillation, this oil has a middle evaporation rate and is primarily sourced from Corsica, the USA, Bosnia, and Croatia. Emotionally, it is said to balance emotions, heal emotional trauma, and enhance intuition and creativity. Patricia Davis describes it as "honey for the psyche."
Therapeutically, Helichrysum essential oil is an analgesic, anti-allergenic, antimicrobial, anti-inflammatory, antifungal, anti-hematomal, antispasmodic, and cicatrisant. It enhances vitality and well-being with its antidepressant and uplifting effects. The oil is non-toxic and non-irritating but should be used in low doses. Its aroma is powerful, rich, sweet, honey-like, fruity, and tea-like with rosy nuances.
GC MS analysis of Helichrysum essential oil
GC-MS Analysis of Helichrysum Essential Oil
The GC-MS analysis of Helichrysum essential oil reveals a diverse composition including terpenes like α-Pinene, β-Pinene, D-Limonene, Eucalyptol, and Caryophyllene, as well as aromatic compounds such as Benzene, Naphthalene, and Azulene.
Key compounds identified are:

Steam distillation
Steam distillation operates on the principle that a mixture of two immiscible liquids, such as water and essential oils, boils at a lower temperature than either of the individual liquids. This happens because the combined vapor pressure of the two liquids is lower, allowing essential oils to be extracted without exposing them to high temperatures that could degrade their components.
In this process, 10.04 grams of Helichrysum flowers are placed in a round-bottomed flask with 135 ml of water. The mixture is heated to 70°C, carefully ensuring that the temperature does not exceed 100°C to prevent degradation of the essential oils. As the mixture boils, steam carries the volatile compounds from the flowers through a condenser, where they cool and condense back into liquid form. After 4 hours, 80 ml of hydrosol is collected, with the flowers turning gray after about 30 minutes of heating, indicating the extraction is in progress. The collected hydrosol is then subjected to Solid Phase Extraction (SPE) and analyzed using Gas Chromatography-Mass Spectrometry (GC-MS) to identify the chemical composition

Microwave distillation
Microwave distillation utilizes microwave energy to heat the plant material directly. The principle behind this method is that microwaves cause polar molecules within the plant to vibrate, generating heat through friction, which leads to the release of volatile compounds.
To begin, 100 grams of Helichrysum flowers are placed in a jar with 500 ml of hot water, allowing the flowers to soak for 10 minutes. The mixture is then heated in a microwave, where the microwave energy selectively heats the water within the plant material, causing it to vaporize and carry the volatile compounds. The vapor passes through a condenser, where it cools and condenses back into liquid form. The obtained hydrosol is refrigerated for one day to cool completely. Subsequently, Solid Phase Extraction (SPE) is performed to separate the volatile compounds from the hydrosol, followed by GC-MS analysis to determine the chemical constituents.

Solid Phase Extraction (SPE) is a technique used to separate volatile compounds from the aqueous phase of the hydrosol. This step is crucial to prevent damage to the apolar column used in subsequent analyses.
To perform SPE, 1 ml of methanol is used for the initial extraction, followed by 1 ml of deionized (DI) water. The total volume of the hydrosol is measured, which in this case is 10 ml. For accurate calibration, six pipettes with different concentrations are prepared. An internal standard, p-Ethyl guaiacol, is added in varying volumes (5 µl, 10 µl, 20 µl, 50 µl, 100 µl, 200 µl) to each pipette.
Once the setup is complete, the flask is prepared to collect the essential volatile compounds. The extraction process involves four rounds, each using 250 µl of a mixture of acetonitrile and methanol. This meticulous procedure ensures the separation and concentration of volatile compounds, readying them for further analysis.

Alcohol extraction of Helichrysum flower
In this procedure, 1.5 grams of Helichrysum flowers are placed into two separate vials. The first vial contains 13.5 grams of 94% alcohol, and the second vial contains 13.0 grams 94% alcohol. The mixture is left to macerate for two weeks, allowing the alcohol to extract the essential compounds from the flowers.
After the extraction period, the mixture is filtered using a syringe filter with a diameter of 0.45 µm to remove any solid particles. This filtered solution is then ready for analysis using the Liquid Chromatography-Mass Spectrometry (LC-MS) method.
For the dilution process, 1 µl of the alcohol extract is mixed with 2 ml of acetonitrile, preparing the solution for precise analysis. This method ensures the accurate detection and quantification of the bioactive compounds present in the Helichrysum extract.

Steam distillation results
The GC-MS analysis revealed variations in the chemical composition between the two distillation methods. Steam distillation identified compounds such as hexanal, acetophenone, and phenol. In contrast, microwave distillation showed higher concentrations of compounds like 1-propanol.
These differences suggest that each distillation method can be tailored to specific applications based on the desired compounds. Steam distillation is effective for identifying a broad range of volatile compounds, while microwave distillation excels at concentrating specific compounds like 1-propanol. Consequently, selecting the appropriate distillation method depends on the targeted chemical compounds for the intended application.
The table shows that many compounds are detected for steam distillation, at 9.2 min retention time carvone is detected, this is a terpene that has antimicrobial, antispasmodic, anti-inflammatory, antioxidant and other properties.

Microwave distillation results
This chromatogram, showing the spectrum at 0.025 µg/ml for a microwave distillation example. This spectrum illustrates the method's ability to detect compounds at low concentrations, highlighting the sensitivity and effectiveness of microwave distillation. And, unlike steam distillation, only 2 compounds are detected.
We detected safrole at 9.5 min is a phenylpropene and has antiseptic and antifungal properties. Safrole, known in the lab as the “frog” molecule, detected during microwave distillation, is of interest in perfumery and aromatherapy, but requires regulated use due to its potential health effects. For carvone, it was also detected at 9.2 minute in higher concentrations in steam distillation (0.0033 mg/L) compared to microwave distillation (0.002mg/mL).The peak at 11 min is only air.
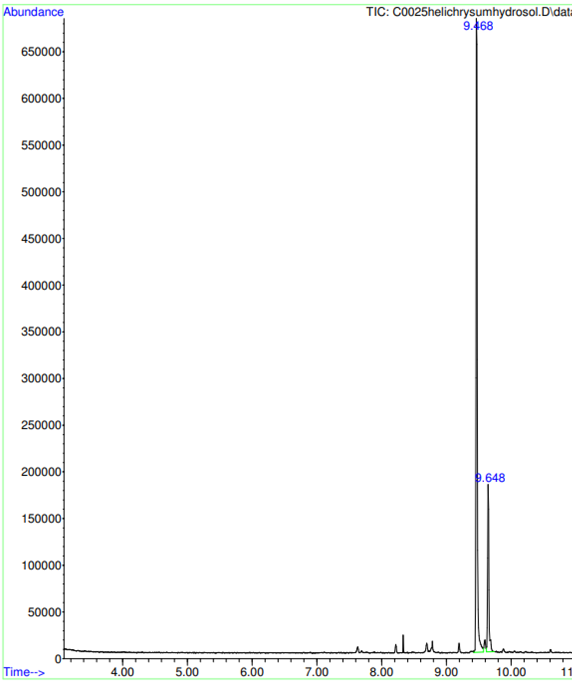
GC-MS analysis of Helichrysum alcohol extract results
GC-MS analysis revealed a wider array of compounds, including Erythritol, Asuccin, Acetamide, Mercury, Methanone, 8-Tetrahydroanthracene, Ethanediimidic Acid, Hexadecanoic Acid, 9-Octadecenoic Acid, Naringenin, and β-Farnesene. This method identified compounds with applications ranging from sweeteners (Erythritol) to solvents (Methanone) and fatty acids (Hexadecanoic Acid). Naringenin was detected again, reaffirming its consistent presence across different analytical methods. The diversity of compounds highlighted by GC-MS underscores the chemical complexity of the extract
Comparing these results with literrature which investigated the chemical composition of Helichrysum italicum, reveals common findings. Both studies identified flavonoids like Kaempferol and Naringenin, confirming their prevalent antioxidant and anti-inflammatory properties in Helichrysum species. The presence of diverse chemical groups such as polyols and fatty acids in both analyses illustrates the plant's broad spectrum of bioactive compounds, supporting its traditional use in herbal medicine. The consistency across different methods and studies reinforces the potential health benefits and complex chemical nature of Helichrysum extracts

LC-MS analysis of Helichrysum alcohol extract results
The LC-MS analysis of Helichrysum extract identified several compounds with FoodB data base: Kaempferol, Naringenin, Apigenin, Oleacein, and Thymidine. Kaempferol and Apigenin, both flavonoids, showed significant antioxidant properties and. Naringenin, another flavonoid, known for its anti-inflammatory benefits. Oleacein, a secoiridoid with potential health benefits, and Thymidine, a nucleoside involved in DNA synthesis, were also detected, emphasizing the diverse bioactive compounds present.

LC-MS/MS confirmed the presence of Apigenin, Naringenin, and Kaempferol, highlighting their health benefits and demonstrating the capability to fragment molecules for component detection.
- Apigenin is a naturally occurring flavonoid found in many fruits and vegetables. Apigenin is known for its antioxidant, anti-inflammatory, and potential anticancer properties.
- Naringenin is a flavonoid present in grapefruits and other citrus fruits.Naringenin offers antioxidant and anti-inflammatory benefits. Naringenin is also found in GC-MS analysis.
- Kaempferol is a flavonoid found in many plants and vegetables. It is recognized for its antioxidant properties and potential health benefits.
Oleacein and Thymidine were not detected in MS/MS, thus their presence in Helichrysum arenarium can not be confirmed.

Conclusion
In conclusion, the hydrosols obtained revealed the presence of various chemical compounds, notably safrole and carvone. It should also be noted that steam distillation, being a gentler method for volatile molecules, enables a greater number of volatile compounds to be detected.
Helichrysum essential oil contains various compounds such as alpha-pinene and caryophyllene, which have anti-inflammatory, bronchodilator, and antimicrobial properties.
For alcohol extraction, various flavonoid compounds were found, which can have a positive impact in the medical field, such as the anti-inflammatory properties of apigenin compounds.
The integration of gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) has significantly enriched our exploration of the therapeutic potential of Helichrysum. These analytical techniques have been instrumental in unraveling the intricate chemical profiles of various Helichrysum extracts, providing valuable insights into their medicinal properties.
GC-MS excels in separating and analyzing volatile compounds, shedding light on the presence of terpenes and aromatic compounds in Helichrysum essential oil. Similarly, LC-MS analysis of the alcohol extract unveiled a spectrum of bioactive compounds, including flavonoids like kaempferol and naringenin, renowned for their antioxidant and anti-inflammatory properties.
Despite challenges in sample preparation and compound characterization, insights gleaned from GC-MS and LC-MS analyses are invaluable, deepening our understanding of Helichrysum's medicinal properties.
GC-MS and LC-MS emerge as indispensable tools for characterizing the bioactive compounds present in Helichrysum extracts, enabling precise identification and quantification. Future research could explore further therapeutic applications of Helichrysum extracts and improve extraction and analysis methods.
